Introduction
A well-organized digital Safety Data Sheet (SDS) library is the cornerstone of chemical safety and regulatory compliance. By adopting a structured taxonomy, leveraging appropriate metadata, ensuring version control, and integrating your SDS repository with broader EHS workflows, you can dramatically improve response times in emergencies, simplify audits, and empower employees with critical hazard information.
How to Organize Your SDS Library (Digitally)
Here are some extended steps that will help you to arrange your SDS library with accuracy:
1. Establish a Clear Taxonomy
Any robust digital library rests on a consistent classification system. A clear taxonomy helps users locate SDSs quickly and ensures uniformity across your organization.
1.1 Define Chemical Categories
Begin by grouping chemicals by broad hazard classes—flammables, corrosives, toxics, oxidizers, compresses gases, and so on. If your operations involve specialized categories (e.g., reactives, polymer precursors), include those as sub-categories.
1.2 Use Departmental or Process-Based Folders
In addition to hazard class, consider organizing sub-folders by department (lab, maintenance, manufacturing) or process (cleaning, coating, synthesis). This dual taxonomy—hazard class plus business unit—ensures both EHS experts and line personnel can find SDSs with minimal clicks.
1.3 Incorporate GHS Pictogram Tags
Tag each SDS’s record with its GHS pictograms. This allows users to filter all corrosive or acute-toxicity materials in a single view, without opening each SDS file.
2. Leverage Metadata for Rapid Retrieval
Metadata—structured descriptors beyond simple file names—turn your SDS library from a static folder into a searchable database.
2.1. Standardize Metadata Fields
At a minimum, capture:
- Product Identifier (as in Section 1)
- Manufacturer/Supplier Name
- CAS Number
- UN Number (for transportation)
- GHS Hazard Statements and Precautionary Codes
- Date of Issue / Revision Date
- Storage Location (if physical copies still exist)
- Physical State (solid, liquid, gas)
2.2. Implement Controlled Vocabularies
Rather than free-text entry, use dropdowns or pick lists for metadata like physical state, hazard class, and department. Controlled vocabulary prevents typos and ensures consistent filtering.
2.3. Enable Advanced Search and Filters
With metadata in place, your SDS management system can support Boolean queries (e.g., “all flammable liquids revised after Jan 2025”) and faceted navigation (e.g., filter by department + hazard class + revision date).
3. Ensure Rigorous Version Control and Audit Trails
Maintaining a single source of truth protects your organization from using outdated information during emergencies or regulatory inspections.
3.1. Assign Sequential Revision Numbers
Each SDS should bear a clear revision code (e.g., “Rev 3.1”) in the metadata and file name. Consistent naming conventions (e.g., “Acetone_Rev3.1_20250515.pdf”) eliminate ambiguity.
3.2. Maintain an Audit Log
Automatically log every upload, deletion, or metadata change with user ID and timestamp. This audit trail is essential for internal compliance reviews and external audits by OSHA or other agencies.
3.3. Archive Superseded Versions
Never delete older SDS iterations. Instead, move them to an “Archive” module accessible only by EHS administrators. Archived copies provide historical context if product formulations change or if regulatory bodies request past data.
4. Integrate with Regulatory Compliance Workflows
Your SDS library should be the hub that connects to hazard communication programs, training platforms, and incident response plans.
4.1. Link to EHS Training Modules
When assigning chemical-specific training in your LMS, embed direct links to the corresponding SDS record. Learners can review Section 2 hazard statements or Section 8 protective equipment requirements in real time.
4.2. Automate Expiry Notifications
Regulatory bodies require SDSs to be kept current. Configure alerts to notify EHS managers when an SDS exceeds a set age threshold (e.g., two years since last revision) or when a manufacturer posts a new version.
4.3. Support Multilingual Requirements
If your operations span multiple countries, ensure the system can store and retrieve SDSs in required languages. Tag and filter by language field in metadata so field operators always access the correct localized version.
5. Adopt Robust Security and Access Controls
Chemical hazard information is sensitive. Proper access controls protect against unauthorized changes and data breaches.
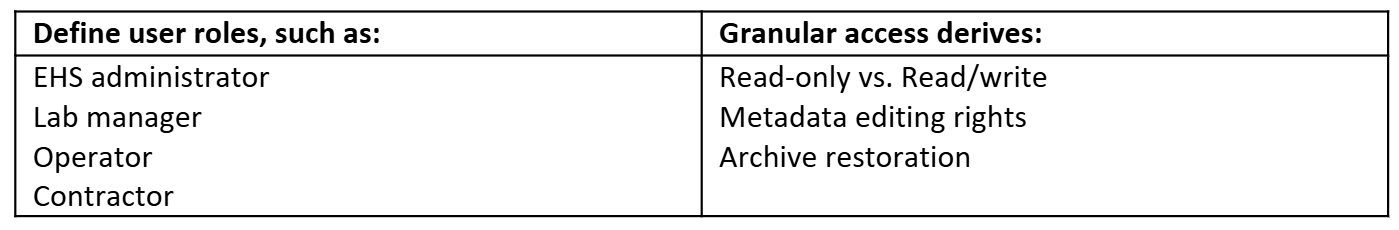
5.1. Role-Based Permissions
5.2. Two-Factor Authentication (2FA)
Require 2FA for users with write or administrative privileges. This extra security layer helps prevent accidental or malicious tampering with SDS records.
5.3. Encryption and Backup
Store SDS files in encrypted repositories, whether on-premises or in the cloud. Maintain daily incremental backups and monthly full backups, with off-site replication to safeguard against data loss.
6. Provide User Training and Support
Even the best system falters without proper training. Empower your workforce to use and maintain the SDS library effectively.
6.1. Develop Standard Operating Procedures
Publish an SOP detailing how to upload new SDSs, update metadata, retrieve records, and archive old versions. SOPs reinforce consistency across teams.
6.2. Host Regular Refresher Sessions
Schedule quarterly training sessions—either live webinars or self-paced eLearning modules—covering system navigation, metadata best practices, and the importance of using current SDSs.
6.3. Establish a Helpdesk Workflow
Implement an internal ticketing system where users can report missing SDSs, request translations, or escalate access issues. Track resolution metrics to continuously improve support.
7. Evaluate and Optimize Continuously
A digital SDS library is not “set and forget.” Continuous monitoring and improvement ensure it meets evolving operational and regulatory demands.
7.1. Solicit User Feedback
Periodically survey stakeholders—lab techs, maintenance teams, procurement, trainers—to identify pain points, feature requests, or indexing issues.
7.2. Monitor Key Performance Indicators
Track metrics such as average time to locate an SDS, number of outdated SDSs discovered during audits, and frequency of training link usage. Use these KPIs to justify system enhancements or budget requests.
7.3. Benchmark Against Best Practices
Review industry guidelines from OSHA, WHMIS, REACH, and ISO standards on chemical information management. Update your taxonomy and metadata schemes to align with new regulatory directives.
Conclusion
By implementing a clear taxonomy, standardized metadata, rigorous version control, seamless regulatory integration, stringent security, and ongoing training, your organization can transform its SDS library into an efficient, user-friendly digital asset. This structured approach not only accelerates hazard communication and compliance but also fosters a culture of safety excellence across all levels of your operations.

Leave A Comment