Introduction:
Whether you work in pharmaceuticals, chemicals, food processing, or industrial manufacturing, outsourcing specialized contract manufacturing organizations (CMOs) helps you bring products to market faster, lower costs, and grow operations. It goes without saying that the role of contract manufacturing organizations or external partners is important. However, there’s one key compliance question every EHS manager and compliance officer in the U.S. must think about: “How do we ensure our contract manufacturing organizations or external partners have access to the right Safety Data Sheets (SDSs)?” Neglecting this issue can lead to serious compliance and safety problems. Outdated or incorrect SDSs can cause improper chemical handling, violations of OSHA’s Hazard Communication Standard, and even accidents at work. To stay compliant and protect your workforce and reputation, ensuring accurate SDS access across your extended network is a top priority.
What is a Contract Manufacturing Organization?
Contract Manufacturing Organizations (CMOs) come in many forms. Some are general-purpose manufacturers, while others specialize in pharmaceuticals, medical devices, or consumer products. The right CMO should align with your product requirements, regulatory standards, and manufacturing goals.
CMOs can generally be classified based on three key factors:
-
Contract structure
-
Supported industries
-
Services, tools, and materials offered
1. Contract Structure
There are several types of contract manufacturing agreements, including:
-
Private-label manufacturing
-
Labor or service subcontracting
-
Component or part manufacturing contracts
-
End-to-end manufacturing partnerships
2. Services, Tools, and Materials
Beyond contract type, CMOs differ in their manufacturing processes, tools, and materials used. These factors directly affect production quality, efficiency, and time-to-market.
3. Supported Industries
Some CMOs specialize in niche sectors. Choosing a manufacturer familiar with your market, customers, and regulatory landscape ensures compliance and long-term success.
Best Practices to Ensure the Right SDS Access for CMOs and External Partners
1. Implement Role-Based Access Control (RBAC)
Not every partner should have access to every SDS. By implementing Role-Based Access Control (RBAC), you can define exactly who can access which SDSs.
Here’s how RBAC supports compliance and data security:
-
CMOs can only access SDSs related to the chemicals they handle.
-
External quality teams can view SDSs for testing or packaging materials.
-
Corporate EHS leaders maintain full visibility through detailed audit logs.
RBAC strengthens security and ensures each stakeholder works from accurate, relevant SDS information — preventing confusion and mismanagement.
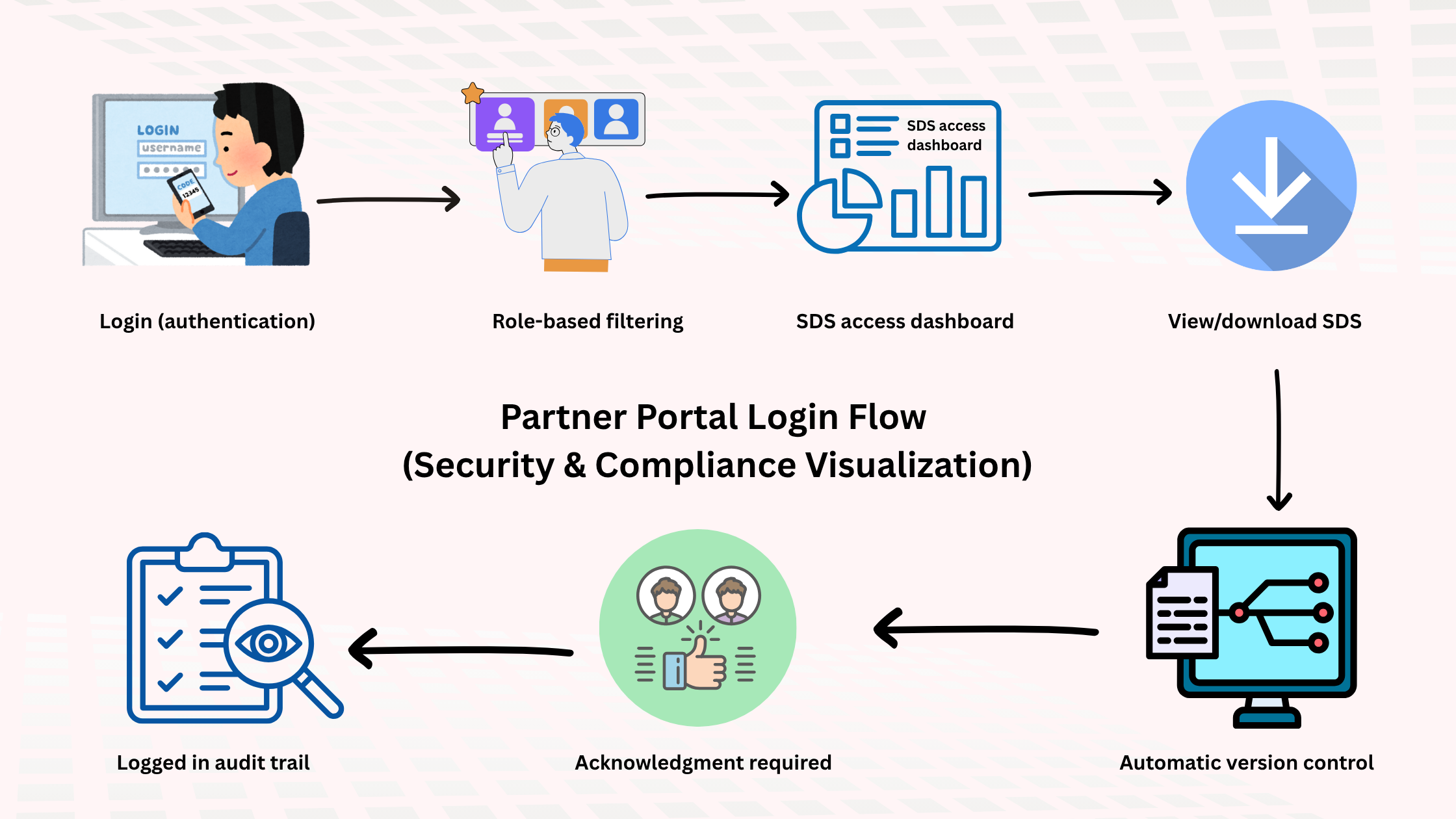
2. Use Secure Partner Portals or LMS Integration
A leading best practice in SDS management is providing access through secure vendor portals or Learning Management System (LMS) integration.
Here’s how it works:
A CMO or external partner logs into your SDS or vendor portal → the system authenticates credentials → and provides SDSs linked to the materials they use.
This approach:
-
Eliminates manual document sharing
-
Reduces email dependency
-
Enables automated tracking and acknowledgment
When integrated with an LMS, the same portal can host chemical safety training and SDS comprehension modules, ensuring partners not only receive SDSs but also understand them according to GHS and OSHA standards.
3. Require SDS Acknowledgment and Tracking
Distributing SDSs isn’t enough — you need proof they’ve been received and reviewed.
Modern SDS management systems include acknowledgment tracking that requires partners to confirm SDS access and understanding before handling materials.
Key features include:
-
Digital read confirmations or e-signatures
-
Automated reminders for unacknowledged SDSs
-
Compliance dashboards showing review completion
These tools create a verifiable chain of custody for safety documentation — invaluable during OSHA, ISO, or internal audits.
4. Conduct Regular SDS Access Audits
Even with automation and access controls, regular SDS access audits are essential to verify that your CMOs and partners remain compliant.
During these audits, EHS leaders should:
-
Review partner access logs to confirm SDS usage
-
Identify outdated or duplicate SDSs
-
Verify that SDSs match the chemicals currently in use
These checks ensure that partners continue to meet your organization’s safety standards and regulatory expectations.
5. Provide Multilingual SDSs for Global Partners
If your CMOs or external partners operate globally, they’ll likely require SDSs in multiple languages.
Your SDS management software should support multilingual compliance with:
-
Automated translation capabilities
-
Country-specific GHS templates
-
Local regulatory formatting (e.g., EU CLP, Japan ISHL, Canada WHMIS)
Even if your company is U.S.-based, offering SDSs in local languages enhances clarity, compliance, and safety culture across your global network.
6. Monitor Access Logs for Transparency
Visibility is essential for compliance. Audit trails and access logs reveal exactly how and when CMOs interact with your SDS system.
Your SDS management platform should record:
-
The name and role of each user accessing an SDS
-
Timestamp of access
-
IP address or location of access
This tracking not only supports OSHA or EPA audits but also provides insights for risk assessment and training improvement.
If certain partners frequently access SDSs for the same chemical, it may indicate confusion or the need for additional training.
7. Embed SDS Access Protocols in Partner Contracts and SLAs
Compliance is most effective when it’s contractual. Every partnership agreement or Service Level Agreement (SLA) with a CMO or external partner should clearly outline SDS responsibilities.
Include clauses that specify:
-
How SDSs will be distributed (e.g., via your SDS portal)
-
Obligations to use only the latest approved SDS versions
-
Timelines for acknowledging and implementing SDS updates
-
Consequences for non-compliance or unauthorized document storage
Formalizing these expectations reinforces shared responsibility for chemical safety and regulatory compliance.
Conclusion:
In the modern industrial supply chain sector, Contract Manufacturing Organizations or External Partners are extensions of your brand and compliance reputation. When they handle hazardous materials on your behalf, their ability to access accurate and current Safety Data Sheets (SDSs) directly impacts your organization’s regulatory standing and risk exposure.
By implementing cloud-based SDS management systems, role-based access controls, automated updates, and formal acknowledgment workflows, you can ensure every partner operates from the same safety framework.
SDS management isn’t just a compliance function—it’s a trust mechanism that unites your internal teams and external collaborators under one goal: safe, consistent, and compliant with chemical handling.
Because when every partner has access to the right SDSs, you’re not just meeting OSHA or GHS requirements—you’re building a stronger, safer, and more transparent supply chain.

Leave A Comment